

Research Interests
Our research programme involves an interdisciplinary approach to create chemistry solutions for reducing atmospheric greenhouse gas concentrations and remediating the environment. The major components of this programme include the (i) design and synthesis of earth-abundant complexes to reduce CO2 and protons, (ii) creation of earth-abundant complexes to oxidise recalcitrant pollutants and valorise biomass, (iii) invention of earth-abundant dyes that can absorb visible light and drive photosynthesis, (iv) integration of new nanomaterials with molecular active sites, and (v) spectroscopic characterisation using static and time-resolved techniques to iteratively improve the novel materials.
Innovated Photosynthesis with Sustainable and Earth-Abundant Nanomaterials
Nanocrystalline catalysts with isolated, defined, molecular active sites can be operationally effective and catalytically active for photosynthesis. Sunlight is an almost inifinte source of energy for innovated photosynthesis. Photosynthesis involves using light ('photo') to make chemicals ('synthesis'). When sunlight is absorbed, charges will separate to form holes and electrons, each of which can separately drive multi-electron catalytic reactions.
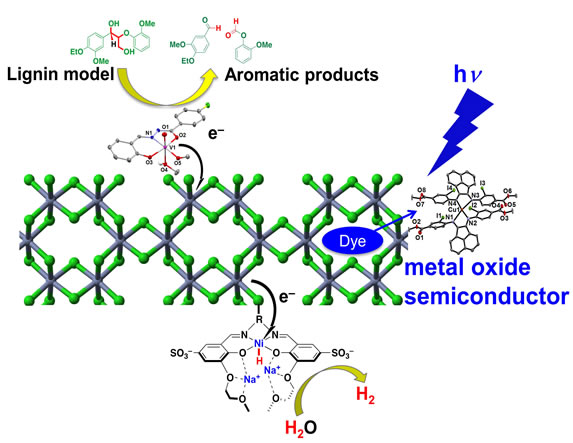
Earth-Abundant Late Transition Metals to Reduce CO2 and Evolve H2
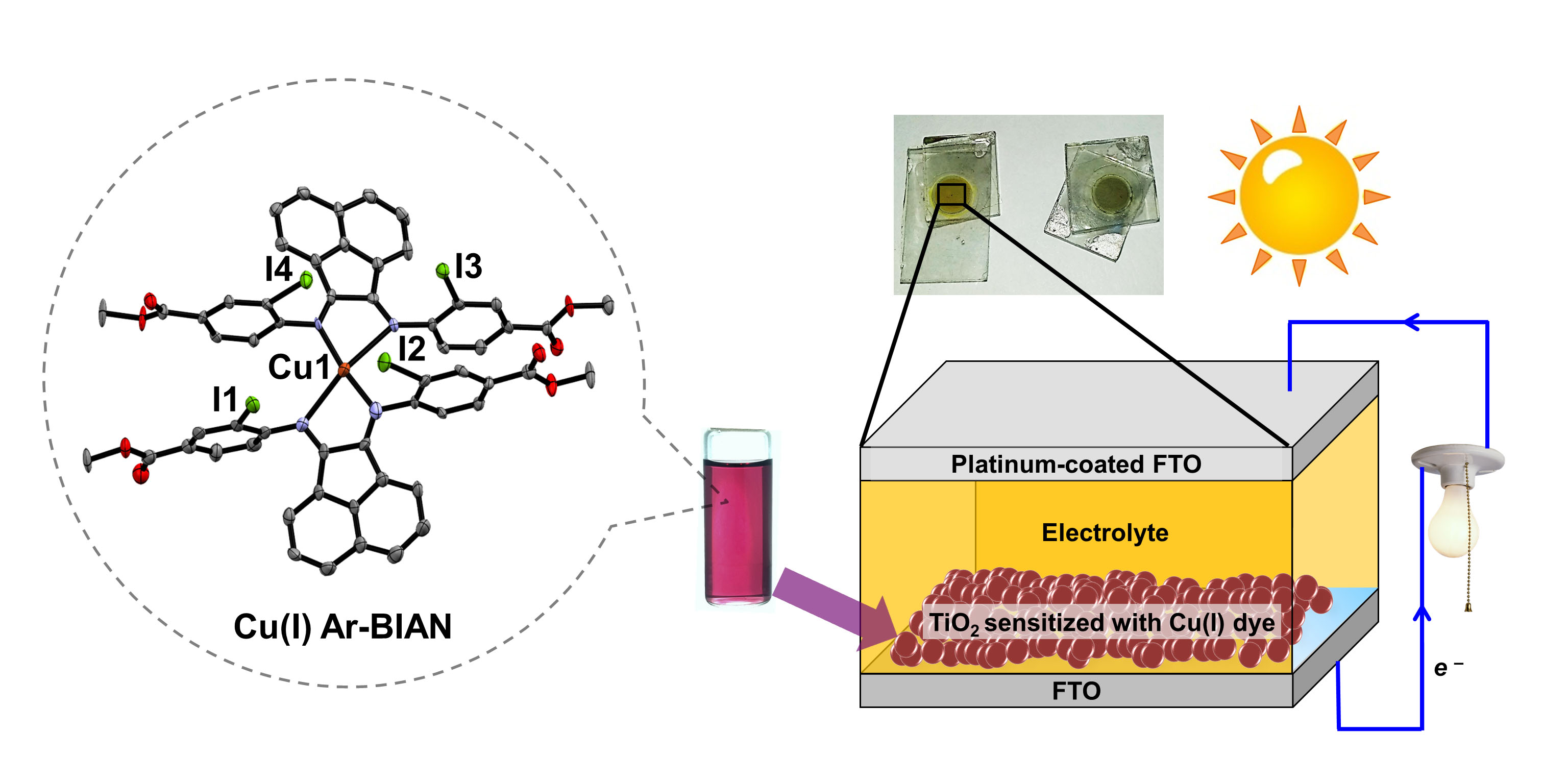
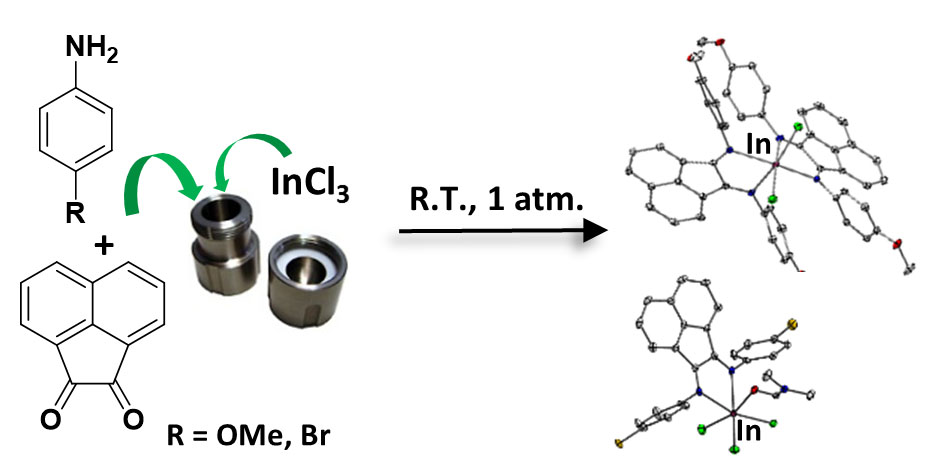
Earth-abundant, first row transition metal compounds have been exploited by Nature for enzymatic reactions and can be ideal catalysts for light-driven CO2 and proton reduction. They can be fully characterised with a suite of techniques including single crystal X-ray diffraction, mass spectrometry, NMR and EPR spectroscopy. However, since the organic supporting ligands of such complexes are often vulnerable to oxidative, light, or thermal damage, anchoring functional transition metal complexes to robust, metal oxide (semi-conducting) scaffolds can improve their operational recyclability.
Degradation of Recalcitrant Environmental Pollutants
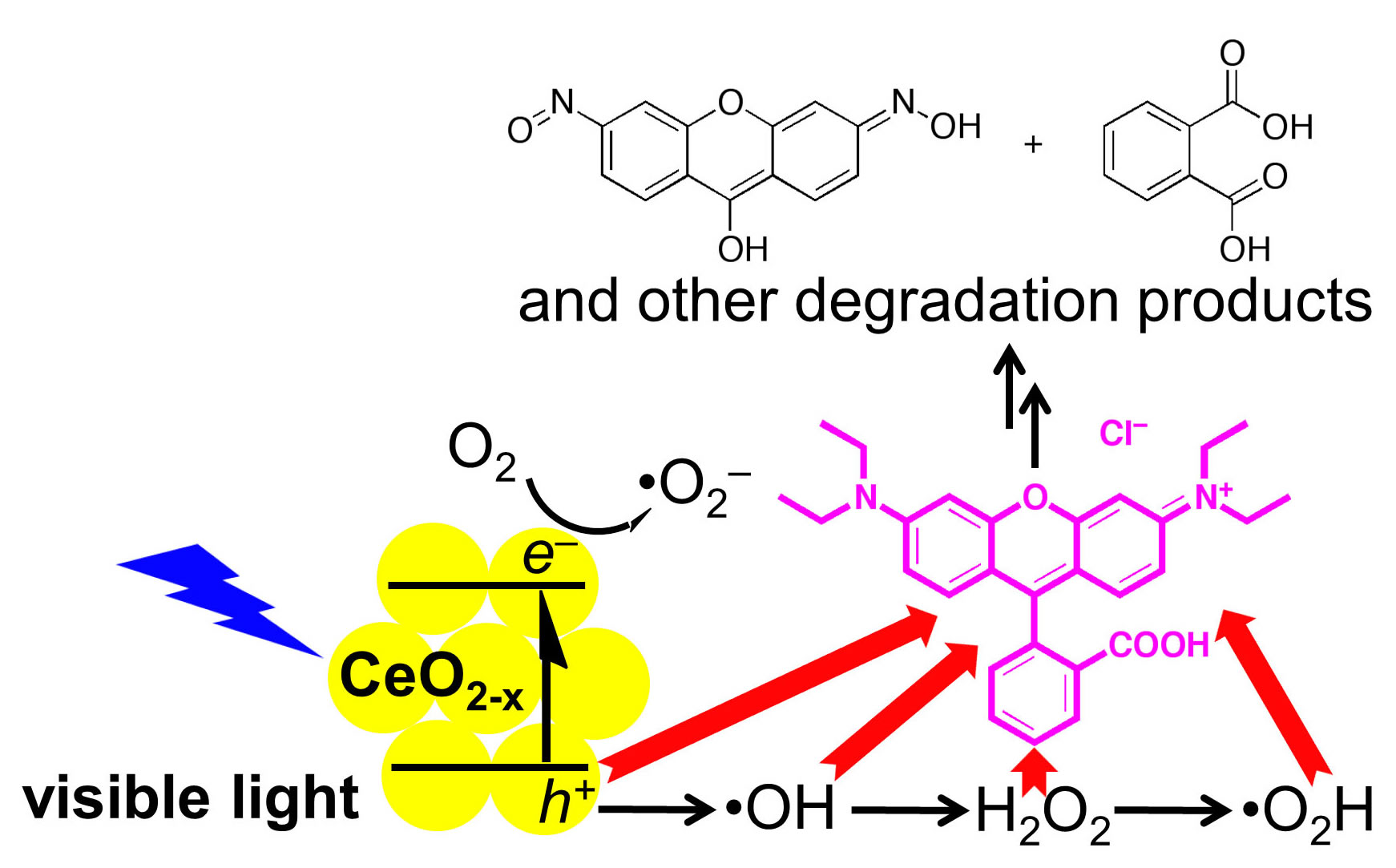
The inventory of harmful, persistent chemicals released into the environment constantly increases. The oxidation of pollutants such as endocrine disruptors can be coupled with CO2 reduction to form a closed electrochemical loop for photosynthesis. Since natural enzymes incorporate high-valent, first-row transition metal complexes to oxidatively degrade organic substrates with remarkably mild, ambient conditions, similar molecular catalysts will be designed and explored.
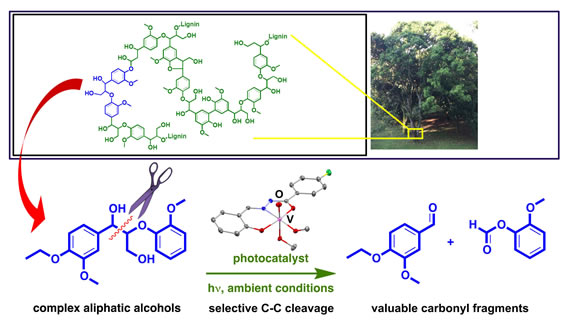
Biomass Valorisation by Selective C-C Bond Photoactivation under Ambient Conditions
Global climate change has intensified the search for sustainable energy and chemical resources. An attractive long-term solution is to profitably convert the lignocellulose in agricultural waste to valuable fine chemicals without harsh chemical methods or combustion. Recovering functionalized phenols and versatile carbonyl compounds by photoredox carbon-carbon bond activations is one such commercially attractive and environmentally sustainable solution.
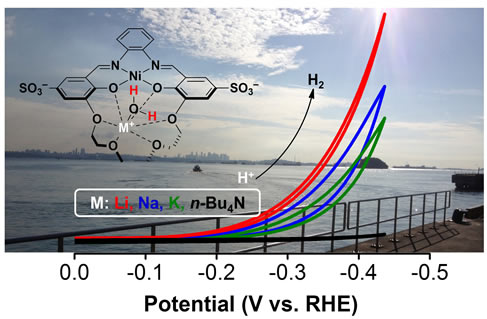
Visible Light Absorption with Earth Abundant Photosensitisers
The solar spectrum at the surface of Earth consists of ~47% visible light and UV radiation, while the remainder is mostly infrared radiation. To efficiently harvest solar energy, photosensitisers that can absorb at least visible light and higher energy will be critical. Robust dyes that consist of earth-abundant elements will be developed and integrated into semiconducting nanocrystals as 'batteries' to drive photosynthesis.
Development of Mesoporous Nanomaterials with Novel Architectures
Earth-abundant semiconducting metal oxides and ternary materials can be stable and absorb visible and UV radiation to drive photocatalysis. Substrates such as CeOx, WOx, and other metal oxides with mesopores or advanced nanoarchitectures have high surface areas with exposed –OH groups that can be used as points of attachment for small molecules. These materials will serve as robust supports but require additional bulk and surface analytical methods such as FT-IR, FT-Raman, diffuse-reflectance UV-vis, X-ray absorption, and X-ray photoelectron spectroscopy.
Systematic Characterisation and Improvements with Time-Resolved Spectroscopy
The intermediates in multielectron photosynthetic reactions are metastable, typically have very short lifetimes, and can only be observed at low temperatures or with time-resolved spectroscopic techniques. Special emphasis will be placed on structure-specific probes such as FT-IR, FT-Raman, and NMR spectroscopy to monitor the kinetics and intermediates of carbon dioxide reduction and O-atom transfer from water. The mechanistic insights drawn from these systematic investigations will aid in the iterative improvement of functional nanomaterials.