|
Research Interests
The
research program in our group integrates organic chemistry, chemical
biology, medicinal chemistry and materials
chemistry. A special emphasis is placed on training and research in
carbohydrate synthesis and on the utilization of this expertise in
addressing problems of medicinal and biochemical significance.
·
Carbohydrate chemistry
·
Glycopeptide and glycoprotein
synthesis
·
Glycobiology
·
Antimicrobial Research
·
Synthetic methods and natural
products
·
Functional materials
Positions Available
Research
Fellow positions (NEW)
Interested candidates should send a summary of research experience,
resume and names of three references to Prof LIU Xuewei (xuewei@ntu.edu.sg) or apply
online:
Workday ID: R00004261
https://ntu.wd3.myworkdayjobs.com/Careers/job/NTU-Main-Campus-Singapore/Research-Fellow--Synthetic-Chemists-_R00004261-1
MCF ID: MCF-2020-0311860
https://www.mycareersfuture.gov.sg/job/sciences/research-fellow-nanyang-technological-university-6c77c29b36a27d7e48ef5d193b8dfcbb
Workday ID: R00004262
PhD
Scholarships in organic chemistry, medicinal chemistry, carbohydrate
synthesis, peptide/protein synthesis and glycobiology
Self-motivated individuals are welcome to join us to work in the area of carbohydrate synthesis, glycoprotein
synthesis, natural product synthesis, and glycobiology. Interested
candidates should contact Prof LIU Xuewei (xuewei@ntu.edu.sg).
Selected
projects
1) Carbohydrate Chemistry
a) Acceptor-controlled Glycosylation and
Carbohydrate Synthesis
Notably, we have developed
the acceptor-controlled glycosylation methods. We have demonstrated the
difference in reactivity of glycosyl acceptors can be employed to
completely drive the stereoselectivity, drawing the parallel comparison
with the commonly practiced donor-controlled glycosylation. This new
concept and method can be applied to efficiently construct different
types of glycosidic bonds, which will certainly contribute to the
synthesis of oligosaccharides and glycoconjugates significantly. Our
contribution was summarized in review papers and book chapters, such as: Acc. Chem. Res. 2018, 51, 628-639; Nat. Commun. 2014, 5, 5051; Angew. Chem. Int. Ed., 2015, 54, 604-607; etc.
i) Hydrogen-bonding mediated glycosylation
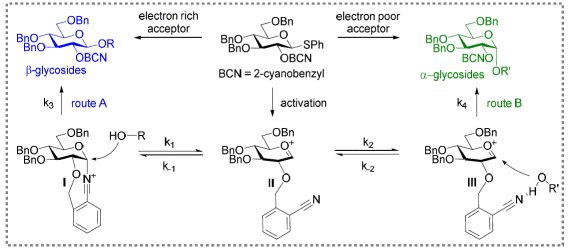
Figure 1: Dual-directing effect of 2-cyano
benzyl ether group on stereospecific glycosylation
ii) Palladium mediated glycosylation
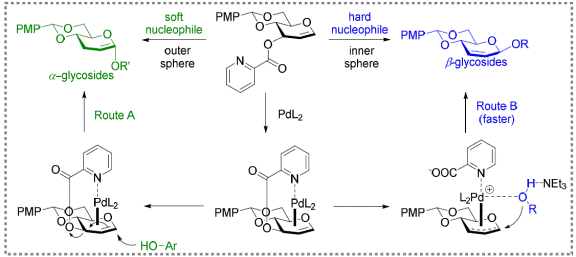
Figure
2: Reversing stereoselectivity via inner sphere or outer sphere
pathway
b) Protection-less/free Glycosylation and
Efficient Carbohydrate Synthesis
Orthogonal protections and
selective deprotection are considered critical for regio- and
stereocontrol in chemical synthesis of complex and diverse
oligosaccharides. The boron-mediated protection-less/free glycosylation
we have developed significantly shortens the synthetic plan, and
laborious orthogonal protection/deprotection steps could be
avoided/minimized. With great advantages and flexibility (Figure 3), our
synthetic protocols can be more elegantly streamlined for both linear
oligosaccharides and branching N-glycans. Some results have been
published, such as: Nat. Commun. 2017,
8, 1146; etc.
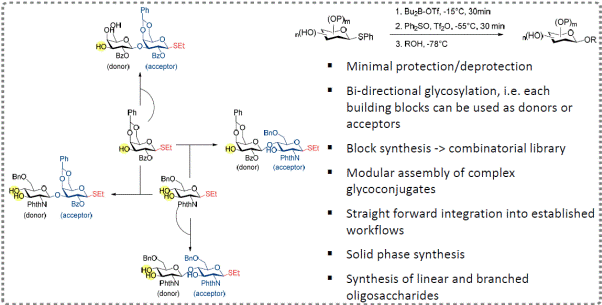
Figure 3: Boron-mediated glycosylation and
efficient carbohydrate synthesis
2) Dual Native Chemical Ligation (dNCL)
and Complex Protein Synthesis
We have developed
conceptually new dual Native Chemical Ligation (dNCL) methods that enable
quick assembly of tagged peptides and post-translationally modified
proteins.
i) Asp-based dual native
chemical ligation and glycoprotein synthesis
We have developed a
practical approach towards N-glycopeptide and N-glycoprotein synthesis
using an auxiliary-mediated dual native chemical ligation (dNCL), as
shown in Figure 4. Results were published: Angew. Chem. Int. Ed., 2016,
55, 10363-10367. Highlighted by
X-MOL.
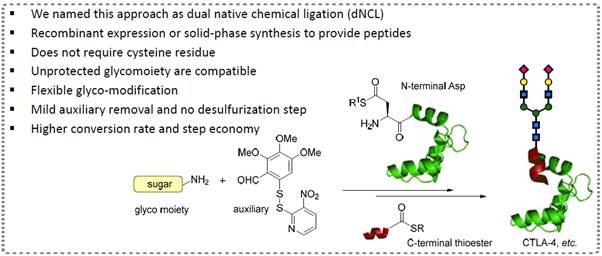
Figure 4: Quick assembly of homogeneous
glycoproteins and glycopeptides via dNCL
ii) Lys-based dual native chemical ligation
and di-ubiquitin synthesis
In collaboration
with Prof Liu Chuanfa’s group, we have designed and prepared the
4-mercaptolysine that can mediate dual chemical ligation at both α- and ε- amines of
lysine via favourable 6-membered ring transition state (Figure 5). It has become a general strategy
and practical method to form branched peptides tagged peptides. Results
were published: 1) Bioorg. Med.
Chem. Lett., 2009, 19, 6268-6271; 2) J. Am. Chem. Soc., 2009, 131, 13592-13593. This work was
highlighted twice by Chemical &
Engineering News (C&EN, Sept. 21, 2009; and Nov. 16, 2009). This
method was applied to
the synthesis of K48-linked di-ubiquitin (Figure 5). Results were
published: Chem. Commun., 2010, 46, 7199-7201. This work was highlighted by Chemical
& Engineering News (C&EN,
Oct. 11, 2010, PP36-37). It
was also selected for Chemical Year in Review (C&EN, Dec. 20, 2010, PP13-17) (Figure 6).
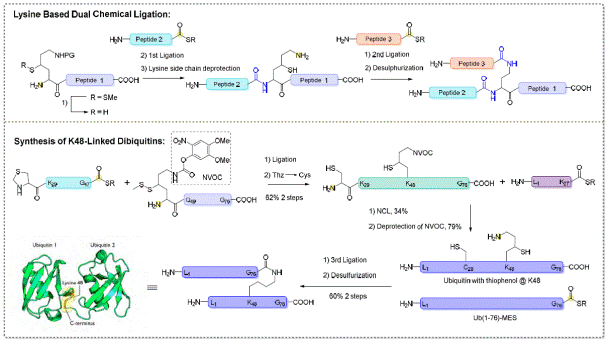
Figure 5: Quick assembly of branching
peptides and di-ubiquitin via Lys-based dNCL

Figure 6:
Our Lys-based dNCL highlighted by Chemical
& Engineering News.
3) Antimicrobial Program: Strategic
Synthesis of Peptidoglycans
Peptidoglycans are unique
structures that are present in all bacterial cell wall, making them an
attractive target for development of wide-spectrum antibiotics. We
developed the first top-down approach to synthesize biohybrid
peptidoglycan oligomers (PGOs) in a highly practical and efficient
manner, starting with the plentiful shrimp shell-derived biopolymer
chitosan. We confirmed the incorporation of PGO into the cell walls of
multiple strains of wild-type bacteria. We have demonstrated that PGOs
can be a powerful tool to study bacterial cell wall biogenesis and
antimicrobial therapeutics. Results were published: Chem. Sci., 2020, 11, 3171-3179.
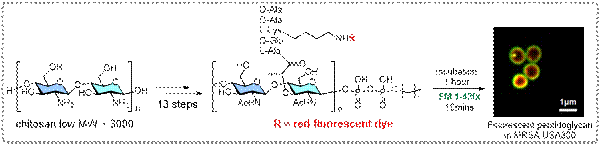
Figure
7: Re-tailoring chitosan as new transglycosylase
substrates for mechanistic studies
4) Sugar-oriented
Synthesis of Natural Products and Drugs
The sweet use of sugars in
organic synthesis often provides unexpected conciseness and new
possibilities. We are strongly interested in economic synthesis of biologically
unique natural products and drugs, starting from sugars. Many results
have been published in top journals: 1) Angew. Chem. Int. Ed., 2011,
50, 12054-12057; 2) Angew. Chem. Int. Ed., 2013, 52, 5134-5137; Highlight in SYNFACTS 2013, 9, 870; 3) Chem. Eur. J., 2014,
20, 405-409; 4) Angew. Chem. Int. Ed., 2014, 53, 10742-10746; 5) Chem. Sci. 2017, 8, 6656-6661; etc.
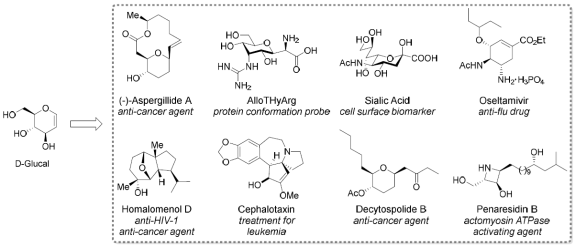
Figure
8: Concise synthesis of natural products and
drugs starting from D-glucal
5) Chemical Glycobiology: Exploring and
Exploiting Carbohydrate-Lectin Interactions
Carbohydrate-lectin interactions
are fundamentally important in various biological processes and play key
roles in cancers, immune diseases, infectious diseases, etc. We are exploring and
exploiting carbohydrate-lectin interactions at the cell surface, by
creating new interdisciplinary approaches and possibilities within
chemistry. For example:
i) “Turn off/turn on” biosensor
We have developed a novel
“turn-off/turn-on” biosensor system to quantify any specific carbohydrate-lectin
interactions (Figure 9). Results were published: 1) J. Am. Chem. Soc., 2012,
134, 15229-15232; 2) Chem. Sci., 2017, 8, 3980-3988; etc.
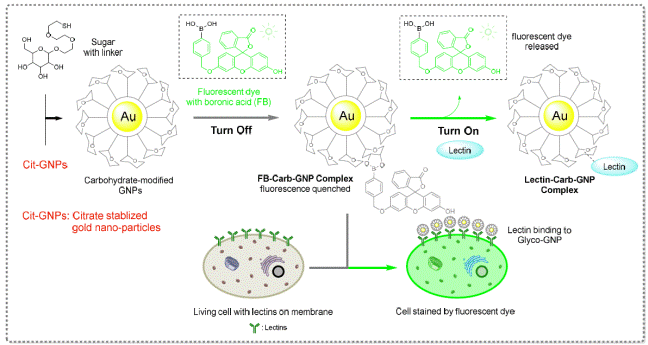
Figure
9: “Turn-off” and “turn-on” system to probe
carbohydrate-lectin interactions
ii) Cell secretion biosensor
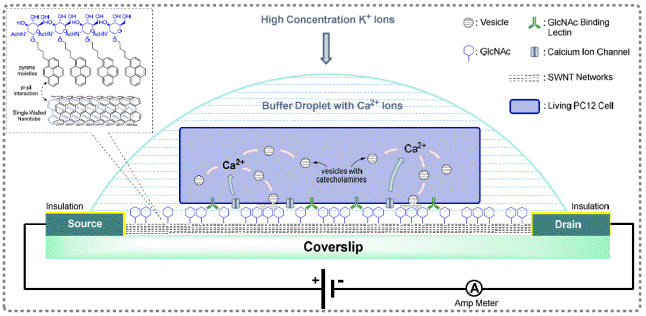
Figure
10: Glycosylated nanotubes interfacing living cells
to detect neuron cell secretion
We have developed the cell
secretion biosensor (Figure 10). Comparing to current methods to detect
exocytosis, our approach provides real-time and non-invasive measurements
from living cells with high sensitivity, high temporal resolution, high throughput and ease of use. The study represents a
great example of how carbohydrates and nanomaterials can bring new
opportunities to biology. Potentially, it can be applied to screen the
side effects of drugs and drug candidates on central nervous system in
developing new drugs. Results were published: 1) Angew. Chem. Int. Ed., 2009,
48, 2723-2726; 2) Chem. Eur. J., 2010, 16, 4533-4540; 3) Chem.
Soc. Rev., 2010, 39, 2925-2934; etc.
|