

Objective: Probing the effects of material characteristics on molecular and cellular immuno-modulation of host response.
Host immune response to implanted biomaterials, which constitute major components of medical devices, drug delivery systems, and tissue-engineered scaffolds, is important for their clinical success. Favorable response facilitates host-device integration, while adverse reaction can lead to compromised function, device failure, and medical complications. Our work focuses on probing the roles of molecular and cellular immune components in the complex interplay between implanted materials and the immune system.
The next step is to deepen our insight into material-induced host immune response, particularly the influence of controlled physiochemical characteristics of material formulation and patterning on the recruitment and activation of adaptive immune cells. Our long-term vision is to leverage this understanding to rationally select and design clinically relevant biomaterials.
Molecular and cellular events occurring after surgical implantation of a medical device
Objective: Rational design of in vivo protease-activated drug delivery systems for management of chronic inflammatory diseases.
A dynamic balance between immune components maintains physiological health. Disruption of this balance can cause chronic inflammation, which requires treatment with anti-inflammatory therapeutics. Current treatments rely on systemic administration or extended release formulations, which often fail to match the temporal dynamics of inflammation, leading to excessive dosing and side effects.
Our research focuses on drug delivery systems that harness disease-specific immunological signals, particularly proteases, to trigger therapeutic release. Long-term goals include expanding these responsive platforms to a wide range of therapeutics (small molecules, peptides, proteins) and evaluating their clinical relevance in conditions such as chronic wounds and arthritis.
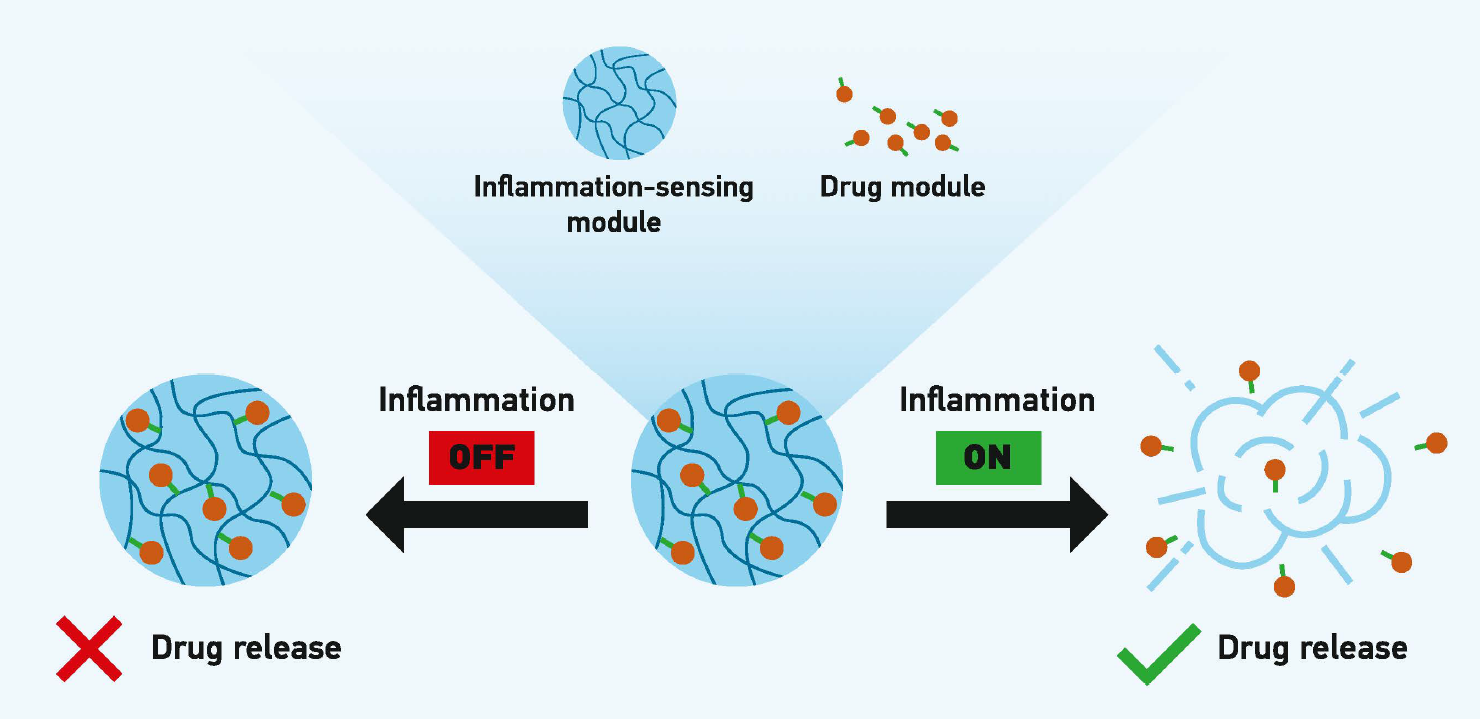
Inflammation-responsive drug delivery
Objective: Rational design of geometrically tailored, immuno-protected microtissues for enhanced viability of cellular therapeutics.
Cellular therapeutics show promise for restoring pancreatic insulin production to treat diabetes. Encapsulation in immuno-isolating hydrogels protects transplanted cells from immune attack without immunosuppressants. However, hypoxia-induced cell death and resulting immune responses often cause graft failure.
Our team pioneers tissue engineering strategies that improve survival of transplanted therapeutic microtissues by (1) assembling them into non-spheroidal geometries to facilitate oxygen diffusion, and (2) positioning them in controlled distributions to minimize aggregation. Looking ahead, we aim to integrate stem cell technology with automated microfabrication for scalable, clinically relevant therapies.

Immuno-isolation and implantation of geometrically tailored therapeutic microtissues