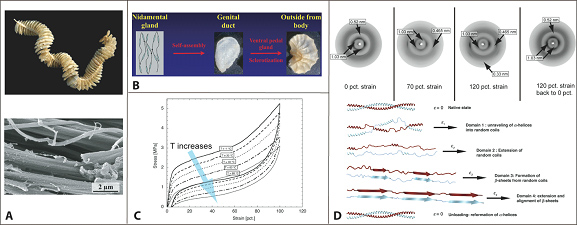
Energy absorbing marine snail egg cases
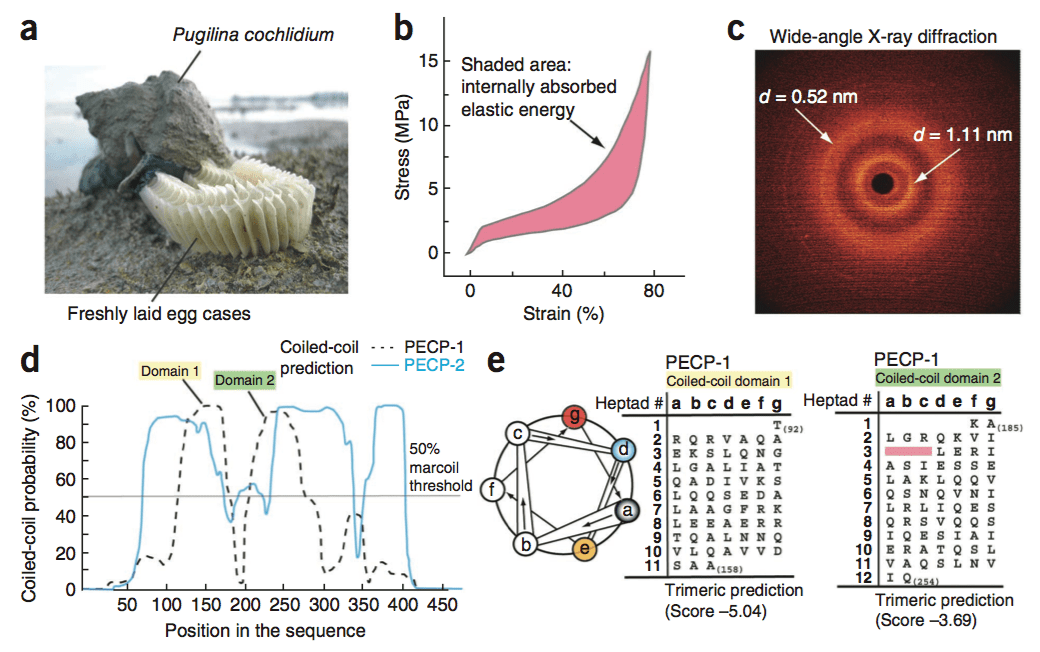
Marine snails lay their eggs inside capsules in the intertidal zone, where they are repeatedly dried and wetted whilst subject to wind, waves, and predators. To protect the delicate embryos from the environmental blows, the capsules are made of a peculiar elastomeric membrane that exhibits a unique combination of structural and physicochemical properties. The capsules feature an initial high stiffness, followed by a pseudo-yield plateau. Re-stiffening then occurs up to the ultimate strains of 150%. Upon unloading, the capsular material fully recovers to its initial dimensions instantaneously; a process accompanied by an impressive hysteresis that allows the dissipation of elastic energy.
Using in situ wide-angle X-ray scattering (WAXS), our findings highlight that the large elasticity is associated with a fully reversible -helical coiled-coil -sheet transition of capsular proteins. In contrast to most elastomers, including bioelastomers, the retractive forces are non-entropic. The elasticity arises from the internal energy changes associated with the transition, a mechanism, which is also responsible for the dissipation of mechanical energy (Nature Materials, 2009).
In the follow-up study, we used a combined RNA-seq-proteomics approach to sequence the egg case proteins (ECPs) that make up the capsules. With this data, we inferred that ECPs are coiled-coil proteins that share strong homology with intermediate filaments (IFs) proteins (Nature Biotechnology, 2013; Chemical Society Review, 2013).
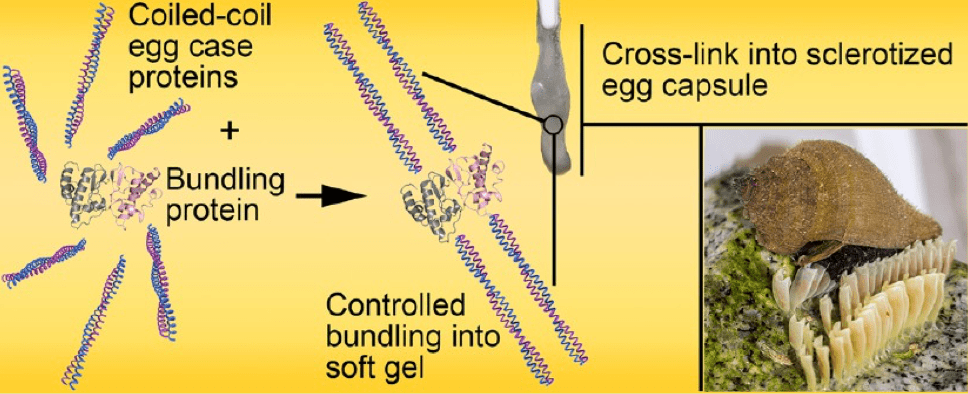
We also established that the IF-like design of ECPs, consisting of coiled coils rods intervened by flexible linkers is common to other marine snail species (Biomaterials Science, 2014), and maybe shared by many other oviparous invertebrates. Following these studies, we have revealed that the bundling protein orchestrates hierarchical self-assembly of ECPs into larger oligomers (Biomacromolecules, 2017), and controls the aggregation of egg case nanofilaments into the macroscopic capsule. This discovery has implications for self-assembly of fibrillar biopolymers and gels since controlled bundling is a common feature associated with their fabrication and also plays a central role in their visco-elastic response.
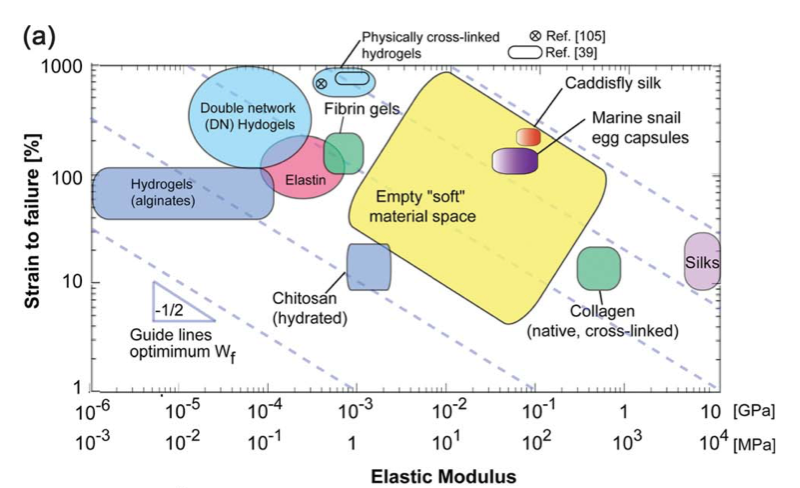
This knowledge is translated into protein engineering and self-assembly approaches to artificially produce these materials (J. Mater. Chem. B., 2015), to use the materials for tissue engineering applications. For instance, mimics of egg case membranes can be used for the encapsulation of delicate tissues or cells. The high mechanical strength, shock-absorbing capability, and biocompatibility of the egg case materials can be used as an advantage during their synthesis. These fibrous materials can be used for regenerative applications that require a combination of extensibility and mechanical strength, for instance, connective tissues.