Particle Technology Lab
Research
Our lab members are currently working on the below projects:
-
Sustainable Manufacturing of Stable Amorphous Drug Nanoplex as a New Nanomedicine Formulation Strategy (Supported by the GSK-EDB Sustainability Partnership, 2013–2017)
-
Process Intensification of Nanocrystalline API Production and Micro-Granulation towards Sustainability (Supported by the GSK-EDB Sustainability Partnership, 2012–2015)
-
Inhalable Biofilm-Targeting Lipid-Conjugated Antibiotic Nanoparticles for Chronic Lung Infection Therapy against P. aeruginosa (Supported in part by the Singaporean-German Research Mobility Scheme, 2012–2013)
-
Co-Formulations of Inhalable Antibiotic Nanoparticles and Mucolytic Agents for Lung Biofilm Infection Therapy (Funded by MOE, Singapore, 2011–2014)
-
Taste Masking of Eurycoma longifolia Extracts by Encapsulation (Industry collaboration, 2012–2013)
Sustainable manufacturing of nanodrugs
Particle
Engineering | Antibiotic
Nanoparticles | Modelling of Turbulent Particle
Laden Flow
Sustainable manufacturing of nanodrugs
 Despite its important roles in the sustainability
enhancement, the current state of nanomedicine manufacturing, by both top-down or bottom-up approaches,
remains far from being sustainable, where preparation methods having high energy expense, heavy use
of solvents, and low mass efficiency are prevalent. Moreover, as a bioavailability enhancement strategy,
the current nanomedicine platform, which employs crystalline drug nanoparticles, has limited impact
because its bioavailability enhancement mechanism relies solely on having faster drug dissolution
rates, but not necessarily higher solubility. Likewise, as a targeted delivery strategy, the
current nanomedicine platform, using drug-encapsulating nanoscale carriers, suffers from low
drug loading capacity, resulting in unsustainable use of carrier materials.
Despite its important roles in the sustainability
enhancement, the current state of nanomedicine manufacturing, by both top-down or bottom-up approaches,
remains far from being sustainable, where preparation methods having high energy expense, heavy use
of solvents, and low mass efficiency are prevalent. Moreover, as a bioavailability enhancement strategy,
the current nanomedicine platform, which employs crystalline drug nanoparticles, has limited impact
because its bioavailability enhancement mechanism relies solely on having faster drug dissolution
rates, but not necessarily higher solubility. Likewise, as a targeted delivery strategy, the
current nanomedicine platform, using drug-encapsulating nanoscale carriers, suffers from low
drug loading capacity, resulting in unsustainable use of carrier materials.
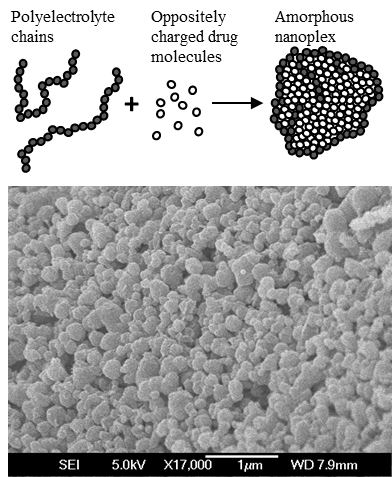
A new nanomedicine platform of stable amorphous drug nanoplex prepared by a sustainable and
versatile method of self-assembly drug-polyelectrolyte complexation has recently been developed
by our group. The amorphous state of the nanoplex facilitates the production of a supersaturating
drug delivery system for bioavailability enhancement purpose, which exhibits multifold higher
solubility than the crystalline counterpart.
By recognizing that (1) most drugs are weak
organic acids or bases that are soluble and ionized in high and low pH
aqueous solutions, respectively, (2) ionized drug molecules are primed
for electrostatic interactions with oppositely charged molecules, and
(3) a majority of orally administered drugs are amphiphilic, we have
exploited in an earlier work the well-established
amphiphile-polyelectrolyte complexation principle to prepare nanodrug
for bioavailability enhancement and targeted delivery purposes. The
nanodrug is prepared by a truly sustainable bottom-up approach of
self-assembly drug-polyelectrolyte complexation to produce stable
amorphous drug-polyelectrolyte nanoparticle complex (or nanoplex). The
said method is (1) simple as it involves only mixing of two aqueous
solutions in ambient conditions, (2) requires minimal energy, (3)
organic solvent free, (4) rapid, and importantly (5) produces high drug
yield (> 90%) and high drug loading capacity (up to 85% w/w).
Particle engineering for inhaled drug delivery
 Dry powder inhaler (DPI) delivery of therapeutic
agents has recently become a subject of very active research with the
FDA approval of the use of spray-dried insulin dry powder aerosols,
Exubera from Pfizer, to treat patients with type 1 and type 2 diabetes.
The FDA approval of Exubera provides a major boost for research
activities in discovering innovative pharmaceutical technologies to
deliver therapeutic agents by inhalation for systemic circulation. In
pulmonary drug delivery, the inhaled particles are subjected to the
phagocytic clearance mechanism in the lung alveolar region by the
scavenging alveolar macrophages.
Dry powder inhaler (DPI) delivery of therapeutic
agents has recently become a subject of very active research with the
FDA approval of the use of spray-dried insulin dry powder aerosols,
Exubera from Pfizer, to treat patients with type 1 and type 2 diabetes.
The FDA approval of Exubera provides a major boost for research
activities in discovering innovative pharmaceutical technologies to
deliver therapeutic agents by inhalation for systemic circulation. In
pulmonary drug delivery, the inhaled particles are subjected to the
phagocytic clearance mechanism in the lung alveolar region by the
scavenging alveolar macrophages.
 Recent studies on pulmonary
nanoparticle deposition suggest that inhaled nanoparticles (<100nm) are
the optimal particle size for alveolar deposition, as well as for
minimizing the lung phagocytosis. However, the application of
nanoparticulate drugs for DPI delivery is not straightforward, as direct
inhalation of nanoparticulate drugs is not feasible due to their
extremely small size. The nanometer size leads to the nanoparticulate
drugs being predominantly exhaled from the lungs, without any deposition
taking place. Moreover, a severe aggregation problem arising from the
small size makes their physical handling extremely difficult for DPI
delivery. To circumvent the above problems, a novel formulation
technique to manufacture large hollow carrier particles of
nanoparticulate drugs has been developed in our group using a spray
drying technique.
Recent studies on pulmonary
nanoparticle deposition suggest that inhaled nanoparticles (<100nm) are
the optimal particle size for alveolar deposition, as well as for
minimizing the lung phagocytosis. However, the application of
nanoparticulate drugs for DPI delivery is not straightforward, as direct
inhalation of nanoparticulate drugs is not feasible due to their
extremely small size. The nanometer size leads to the nanoparticulate
drugs being predominantly exhaled from the lungs, without any deposition
taking place. Moreover, a severe aggregation problem arising from the
small size makes their physical handling extremely difficult for DPI
delivery. To circumvent the above problems, a novel formulation
technique to manufacture large hollow carrier particles of
nanoparticulate drugs has been developed in our group using a spray
drying technique.
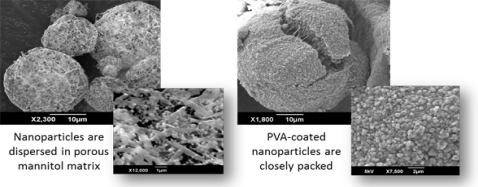
For thermally sensitive materials, however, spray drying is not a suitable method to convert nanoparticulate suspensions into dry powder due to the high temperature involved. To overcome this hurdle, the spray-freeze-drying technique can be utilized to prepare re-dispersible nanoparticulate aggregates. Lyoprotective excipients are employed to prevent aggregation of the nanoparticles, resulting in different particle morphologies.
Antibiotics nanoparticles
The inhalation of nanoparticles represents an attractive delivery route, because of the avoidance of the first-pass metabolism, ease of administration, and fast drug absorptions due to the lungs’ high surface area and thin epithelial layer. Specifically, antibiotic-loaded nanoparticle formulations have been actively researched as inhaled therapeutic carriers, because of the prospects of having (i) a targeted delivery by surface functionalization of the carrier, and (ii) a sustained delivery by drug encapsulation in the carrier.
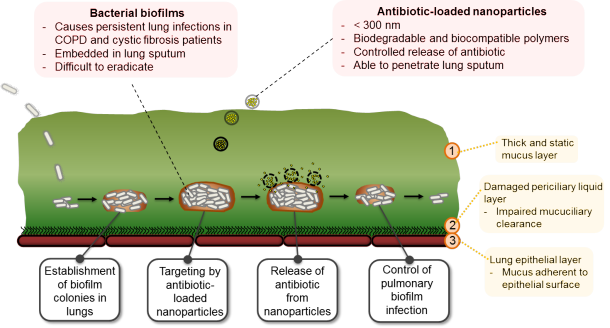
Antibiotic-loaded nanoparticles are highly applicable in the treatment of persistent pulmonary infections, such as cystic fibrosis (CF) and chronic obstructive pulmonary diseases (COPD), a disease suffered by more than 50 million people worldwide. Bacterial biofilm colonies present in the lungs of CF and COPD patients are surrounded by thick stationary sputum notorious for binding and deactivating antibiotics, thereby negating the therapeutic effects of the inhaled antibiotics. Encapsulation of antibiotics in nanoparticles overcomes the problem of antibiotic deactivations as it prevents interactions between the antibiotics and the sputum contents.
Modelling and experimental investigation of particle-laden flows
Turbulent fluid-particle flow is a cornerstone for many energy-related industrial processes, such as in fluid catalytic cracking (FCC) riser reactor, pneumatic transport of particulates, and coal combustion/gasification. Despite its widespread industrial applications, many of these processes do not operate at their optimal condition due to a lack of understanding in the intricate flow phenomena that occur in a turbulent fluid-particle flow. This lack of understanding in the particulate flow phenomena leads to difficulties in the design, optimization, and scale-up of such processes. These tasks would typically require reliable simulation tools that can accurately predict the particulate flow behavior in large-scale industrial processes.
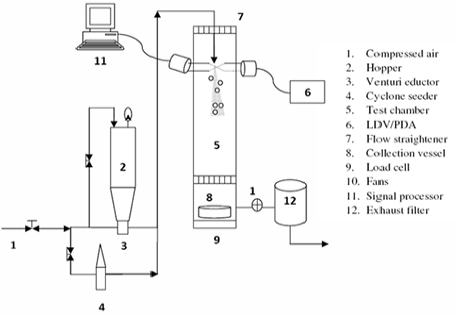
For this reason,a continuous advancement of two-phase flow CFD model that can capture both the micro and macroscale particulate flow phenomena is critical for improving the industrial processes. In conjunction with the modeling work, non-intrusive flow measurements, where multiple flow variables are measured simultaneously, are needed to develop and validate the CFD model.